Acute, severe traumatic spinal cord injury is a devastating condition. Other than fixing the spine, neurosurgeons have little to offer these patients to improve their outcomes. Neurosurgical discussions focus on the surgical approach to be used and whether early surgery makes a difference. Current guidelines suggest maintaining mean arterial pressure at 85–90 mmHg for all patients a week after injury, to improve spinal cord perfusion, but with little supporting evidence. Here, we propose a different way of looking at the problem.
Because the brain and the spinal cord are composed of the same cell types, it is reasonable to assume that both organs have similar response to injury. This means that the injured spinal cord, like the injured brain, will swell and become compressed against surrounding structures including dura and bone. The concepts of intracranial pressure, cerebral perfusion pressure and pressure reactivity, which were developed for managing brain injured patients in the intensive care unit, should also apply to spinal cord injury; the corresponding concepts for spinal cord are intraspinal pressure, spinal cord perfusion pressure and spinal pressure reactivity.
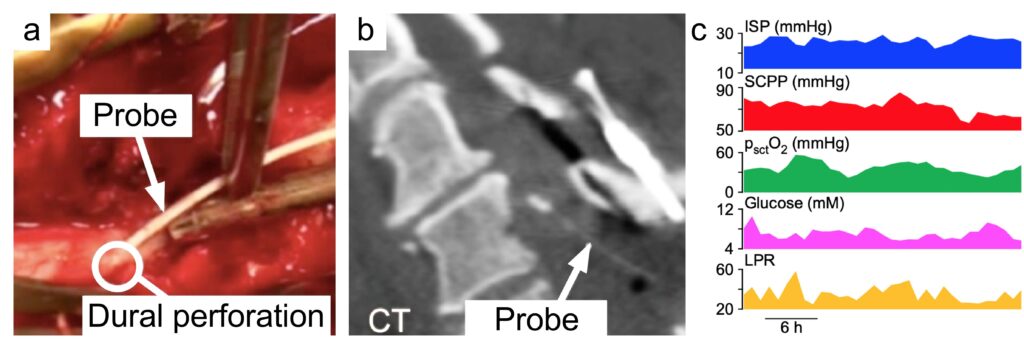
To monitor these physiological parameters, we insert a pressure probe intradurally at the site of injury. We also insert a microdialysis catheter and an oxygen electrode to monitor spinal cord metabolism and tissue oxygen. These probes (pressure, microdialysis, oxygen) are inserted during surgery to fix the fractured spine and the insertion is done through a posterior approach (Figure). In effect, this setup achieves multimodality monitoring for spinal cord injury, analogous to multimodality monitoring for brain injury. Such monitoring allows intensivists to detect insults to the injured cord early and intervene to prevent secondary spinal cord damage.
There is now evidence that intervention to increase the spinal cord perfusion pressure may improve the sensory level, limb motor score as well as urinary bladder and anal sphincter functions. The average intraspinal pressure and the average spinal cord perfusion pressure over the monitoring period correlate well with long-term outcome. Such monitoring also produced novel concepts, e.g. not only very low but also very high spinal cord perfusion is detrimental and the optimum spinal cord perfusion pressure varies between patients, which suggests individualized treatment rather than having the same blood pressure targets for all. Recently, we found that fever causes metabolic stress at the injury site and fever burden is an independent prognostic factor.
Another interesting observation is that, after spinal cord injury, the injured spinal cord becomes compressed against the dura and, therefore, effective decompression may require duraplasty in addition to bony decompression. We have thus set up a randomized, controlled trial to determine whether bony decompression plus duraplasty improves outcome after severe spinal cord injury compared with bony decompression alone (https://clinicaltrials.gov , study no. NCT04936620).
For details about the technique of monitoring intraspinal pressure, spinal cord perfusion pressure, tissue oxygen and tissue metabolism from the injured spinal cord in the intensive care unit see our recent review (https://link.springer.com/article/10.1007/s13311-019-00820-6).