Global neurosurgery is often viewed through a dichotomous lens. Some see it as a way to give, while others question its value. One thing is certain, individuals with experience in global neurosurgery diversify the collective experience of the neurosurgical profession. I believe that creating novel solutions to adapt neurosurgical treatment options to low-resource environments will advance the standard of care universally.4
Over the past ten years, I have had the opportunity to work across Latin America as an interpreter for medical teams and distribute therapeutic devices to children with disabilities. Many of my patients have comorbid neurosurgical conditions, like hydrocephalus. Those with shunts are often at high risk of morbidity and mortality from shunt failure, due to difficulty detecting failures early and reliably. Often, because they live in rural communities, by the time they experience unequivocal symptoms of elevated intracranial pressure (ICP), it is often too late for effective treatment. This problem motivated the development of a new shunt focused on shifting the diagnostic paradigm from reactive (symptom-based identification) to proactive (pre-symptom identification).
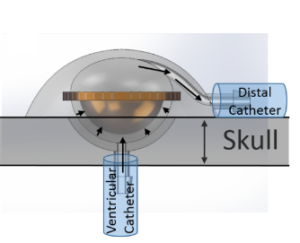
Ventricular shunts are life saving devices that remain the mainstay of worldwide treatment for hydrocephalus, despite the fact that they fail often from blockages. Current diagnostic methods to evaluate for obstruction are potentially hazardous, always costly and require utilization of resources such as plain radiographs, CT, MRI and even nuclear isotopes. In resource-poor settings, these factors can lead to untimely diagnosis, thus increasing morbidity and mortality. This simple fact incentivizes a re-examination of current methods. A new shunt design in the works provides real-time feedback about flow through the shunt using more universally available resources like smartphones and ultrasound (U.S.)1 to monitor valve actuations as a proxy for monitoring CSF flow (Figures 1&2)1.
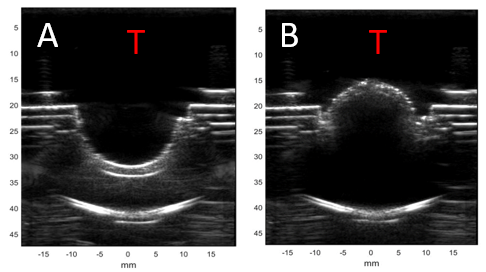
Using ultrasound to detect real-time shunt flow will expand access and provide physicians safer and more cost-effective means to diagnose shunt failures. Ultrasound is a technology that is rapidly being adopted and utilized by physicians around the world to diminish reliance on more multiplanar imaging modalities. For example, portable ultrasound systems can now connect to a physician’s smartphone, converting them into a mobile point-of-care ultrasound (POCUS) system for around 2,000 USD.
With 3.3 billion smartphones in use globally, they are nearly ubiquitous around the world, even in developing communities and low-income households. In the communities that I have visited, in mountainous villages and homes constructed from cinder blocks, I have found that at least one member of the household owns a smartphone. With this in mind, an implantable sensor can be developed for a shunt system that can transmit real-time data about shunt activity to a smartphone app. This app could be used to alert users of shunt drainage abnormalities prior to the development of more extreme symptoms. It will also empower users to reliably and proactively respond to shunt failures. This will decrease patient time to treatment and caregiver anxiety related to any uncertainty identifying shunt failures.
Experience in global neurosurgery can help identify needs and encourage projects – something that has helped inspire and shape my own interest in the field of neurosurgery. The shunt project is still in its infancy, but there has been promising success with miniaturizing benchtop models to “implantable scale” for mice and monitoring the valve with ultrasound and various implantable sensors. The hope is to transition this project from benchtop to in vivo models during the coming years (coincident with my residency). Personally, the opportunity to discuss this project with faculty and residents across the country during residency interviews and their ensuring excitement and support has energized me.
I hope that we all will continue to take advantage of diverse opportunities within the wide world of neurosurgery, particularly in global neurosurgery, and will be stimulated to bring new ideas and solutions to issues that affect patients.
References
[expand title=”View All”]
1. Sippel, S., Muruganandan, K., Levine, A., & Shah, S. (2011). Review article: Use of ultrasound in the developing world. International Journal of Emergency Medicine, 4(1). doi: 10.1186/1865-1380-4-72
2. Mitchell, S., Grangard, G., Kahouli, W., Dalldorf, C., Crain, A., Lee, E., … Bauer, D. (2019). Snap-valve cerebral shunt design for intracranial pressure operation and ultrasound visualization. Medical Engineering & Physics, 66, 1–11. doi: 10.1016/j.medengphy.2018.12.024
3. Naftel, R. P., Tubergen, E., Shannon, C. N., Gran, K. A., Vance, E. H., Oakes, W. J., … Wellons, J. C. (2012). Parental recognition of shunt failure: a prospective single-institution study. Journal of Neurosurgery: Pediatrics, 9(4), 363–371. doi: 10.3171/2011.12.peds11291
4. Warf, B. C. (2017). Growing Brains: How Adapting to Africa Advanced the Treatment of Infant Hydrocephalus. Neurosurgery, 64(CN_suppl_1), 37–39. doi: 10.1093/neuros/nyx246
[/expand]
[aans_authors]







