Revascularization Procedures for the Treatment of Moyamoya Disease
Case Description
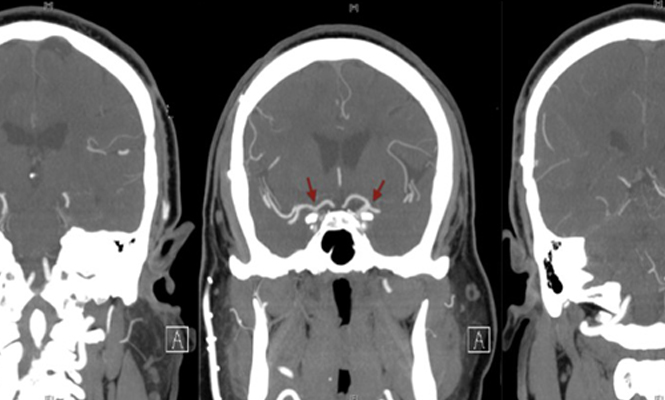
A 36-year-old, right-handed, Indian-American gentleman with no significant past medical history presented in 2006 with right hand weakness secondary to a lacunar infarct in the left middle cerebral artery (MCA) territory. Magnetic resonance angiography (MRA) showed significant bilateral stenosis of the internal carotid arteries (ICAs) and an abnormally large amount of contrast dye in the small cerebral vessels, suggestive of Moyamoya disease (MMD). Diagnostic cerebral angiography (DCA) was consistent with bilateral internal carotid stenosis (left worse than right) distal to the ophthalmic arteries and anterior cerebral cortex perfused by the posterior circulation. Extensive small vessel collateralization was seen, confirming the diagnosis of MMD (Figure 1).
[aans_poll id=”3503″ view=”prompt”]
Discussion
MMD (Japanese for ‘puff of smoke’) is a chronic, non-atherosclerotic, occlusive cerebrovascular disease characterized by unilateral or bilateral progressive stenosis, or occlusion, of the supra-clinoid internal carotid arteries with prominent small vessel arterial collateralization at the cranial base (1). MMD is the most important cause of non-atherosclerotic intracranial arterial disease in countries such as Japan and Korea (1). The precise etiology or causative factor of MMD is unknown, but there may be a genetic predisposition with a strong familial tendency. The RNF213 gene on the short arm of Chromosome 17 is implicated, especially in Asian populations, as a possible susceptibility factor (1). The incidence of MMD is estimated at approximately 10/100,000 in Asian countries and is highest among the Japanese and Korean populations. This is slightly higher than that observed in the U.S., and even in this country, persons of Asian descent are at higher risk for MMD than Caucasian, Hispanic and Black populations. Worldwide, the incidence peaks in the 10-20-year and 35-50-year age groups and is more common in women. A family history of MMD is noted in 10 to 15 percent of patients (1).
Repeated ischemic events, transient or permanent, are the hallmark of MMD. In addition to the known vessel lumen obliteration and occlusion, hemodynamic factors have a significant influence on the occurrence of these events. A decrease in arterial PaCO2 due to hyperventilation may lead to vasodilatation of normal cerebral vessels and misery perfusion due to a “steal” phenomenon in vessels affected by MMD. Even relatively innocuous activities, such as stress, fatigue, dehydration or infection, may precipitate an ischemic event in MMD patients. Ischemia is mainly observed in the anterior circulation with aphasia, hemiplegia or hemisensory loss although posterior circulation involvement is observed in late stages of the disease and may portend a poor prognosis (1).
In addition, the topography of the infarct may not always follow normal vascular territories due to the diversity of collateral circulation formation (1). One third of patients may present with primary intracerebral hemorrhage due to the friable nature of the collateral vessels or rupture of pseudoaneurysms in these abnormal vessels (1). Intraventricular and callosal hemorrhage also tends to more common in MMD patients who suffer an intracranial hemorrhage compared to patients who suffer a primary intracerebral hemorrhage due to hypertension or amyloid angiopathy (1). An abnormal and prominent anterior choroidal artery, or microaneurysm, of this vessel may be the source of these hemorrhages. Similarly, cerebral microbleeds in the deep periventricular regions of the brain may be due to the fragility of collaterals around the anterior choroidal or posterior communicating arteries and represent radiographic harbingers of future intraventricular hemorrhages (1). Other clinical manifestations of MMD include seizures, cognitive impairment or intellectual decline, or involuntary movements in young patients and the occurrence of migraine-type headaches that may be due to cerebral hypoperfusion. Ischemic symptoms and signs tend to be more common in children and young patients, and intracerebral hemorrhage is more common in older age groups.
The low incidence and phenotypic variation of MMD make understanding its natural history difficult. A ‘classic’ progression of MMD was described in 1969 by Suzuki and Takaku (2). They recognized six distinct stages as shown in the table below.
Despite its widespread usage, the Suzuki classification’s role in guiding treatment and treatment response is unclear. There are no established guidelines linking a certain stage with a particular treatment. Cerebral digital subtraction catheter angiography is the test of choice, but bilateral cases may also be diagnosed by MRA. High-resolution vessel-wall magnetic resonance imaging (MRI) demonstrates concentric narrowing of the outer diameter of affected vessels; three-dimensional (3-D) constructive interference in steady-state (CIISS) is particularly sensitive in detecting these vascular changes. With angiography, peri-procedural ischemic complications may occur and adequate hydration, particularly in pediatric patients, is essential (1).
Cerebral hemodynamic imaging modalities are currently being studied for predicting long-term outcomes in surgical candidates. CT-perfusion, transcranial Doppler, single-photon emission CT and arterial spin-labeling MRI are all modalities that can assess cerebrovascular reserve (3). This is done by measuring cerebral blood flow before and after the use of a vasodilatory stimulant – acetazolamide challenge, inhaled carbon dioxide or hypoventilation. Additionally, positron emission tomography (PET) is used to assess oxygen extraction fraction in cortical areas that have maximized their cerebrovascular reserve (3). These studies are routinely used in pre-operative work-up, but their impact on surgical options and outcomes is not well understood. Finally, 3-D digital subtraction angiography is being studied as a surgical planning tool to identify ECA/ICA vessels and reduce craniotomy size (3).
The goal of treatment of MMD is to alleviate symptoms and prevent future TIA/stroke; there is no known treatment that stops or reverses the vasculopathy. Treatment options can be initially divided between nonsurgical and surgical revascularization. Several nonsurgical regimens exist utilizing the following drugs either alone or in combination: aspirin, other antiplatelet agents, mannitol, antibiotics, steroids, calcium-channel blockers and low-molecular-weight dextrans. Patients are encouraged to avoid dehydration for adequate cerebral perfusion, and blood pressures must be normotensive to decrease risk of stroke.
Several surgical techniques can be divided into direct and indirect revascularization. Direct revascularization involves the surgical anastomosis of an external carotid vessel to an intracerebral vessel, with or without the use of an arterial graft, providing extracranial retrograde flow to the circle of Willis. Most commonly, the superficial temporal artery is anastomosed to an M3 or M4 branch of the ipsilateral hemisphere, although middle meningeal and occipital artery bypasses have also been described. These techniques provide immediate revascularization for the otherwise ischemic brain. Direct revascularization is a technically difficult procedure, especially in children where the caliber of arteries may be too small to directly operate on. It is also limited to the MCA territory and may not be the best option for patients with simultaneous significant ACA or PCA involvement. Hyperperfusion syndrome may follow direct revascularization procedures, but permanent neurologic sequelae are rare.
Similarly, the goal of indirect revascularization is to provide external carotid blood for intracerebral perfusion. However, these procedures create a tissue environment that promotes intracranial angiogenesis rather than direct anastomosis of two vessels. Many techniques exist, including encephalo-myo-synangiosis, encephalo-duro-arterio-synangiosis, pial synangiosis, encephalo-pericranio-synangiosis, dural inversion, omentum transplantation, gracilis muscle transplantation, burr hole drilling, and modifications of the above. In general, these options place highly vascularized tissue in direct contact with the brain’s surface, promoting anastomosis of extracranial and intracranial vessels. They do not depend on vessel caliber and are less technically difficult resulting in lower operative and anesthesia times. They are also not limited to a single major arterial division; both anterior and posterior circulatory deficits can be primarily assessed. Also, multiple indirect revascularization techniques can be combined and used on the same hemisphere. Despite these benefits, collateralization can take weeks to months, putting the patient at risk for stroke before perfusion becomes adequate. Inconsistent angiographic responses have also been demonstrated in adults compared to children, although clinical outcomes were not statistically significant.
While there are no randomized control trials (RCTs) comparing nonsurgical treatment to surgical revascularization, stroke rates in respective studies of these two modalities suggest the superiority of surgical intervention. Without surgical revascularization, the majority of pediatric and adult patients will suffer from at least one ischemic or hemorrhagic stroke within five years of diagnosis. On the other hand, revascularization has been shown to significantly decrease stroke rate in both pediatric and adult patients.
In 2009, researchers from Stanford gathered data from 233 adults undergoing 389 procedures and 96 pediatric children undergoing 168 procedures (mean follow-up one and a half years) (4). Direct revascularization was used in 95.1 percent and 76.2 percent of adult and pediatric patients, respectively. Peri-operative morbidity and mortality rates were 3.5 percent and 0.7 percent, respectively. In 264 patients undergoing 450 procedures with long-term follow-up, the five-year stroke rate was 5.5 percent (4). This particular study represented the largest moyamoya sample size to date, and several other smaller studies have reported five-year stroke rates from 5 percent to 22 percent (3,5). Despite several observational studies, there are no RCT’s comparing the different surgical options. Additionally, outcomes from direct, indirect and combined revascularizations are typically combined. Furthermore, indirect and combined revascularization procedures within individual studies often combine different techniques. No particular procedure has been proven to be most effective. This has made choosing a surgical option an individualized decision based on pros, cons and surgeon preference.
Clinical and radiological findings guide the indications for surgery, but there remains some variance. Some recommend surgery at onset of ischemic complications while others recommend surgery with radiological evidence of decreased cerebral perfusion alone via SPECT or PET scans. Also, MMD is typically a bilateral disease, but patients often progress somewhat asymmetrically with one hemisphere being more affected than the other. A minority of MMD patients have the unilateral variant, in which the contralateral vessels go unaffected. There is no clear consensus on when to operate on the contralateral hemisphere in either of these situations. Finally, differences in the management of MMD – MMD in the setting of Down syndrome, sickle cell anemia, neurofibromatosis type 1 or radiation – have not been determined.
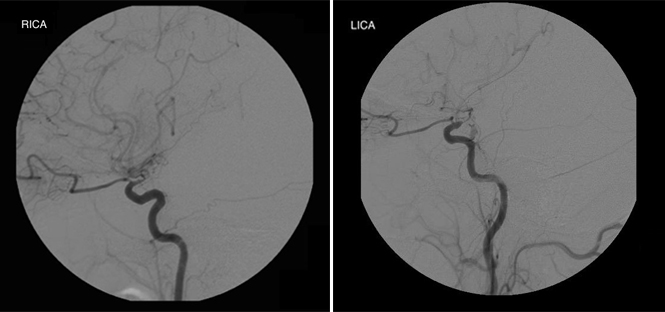
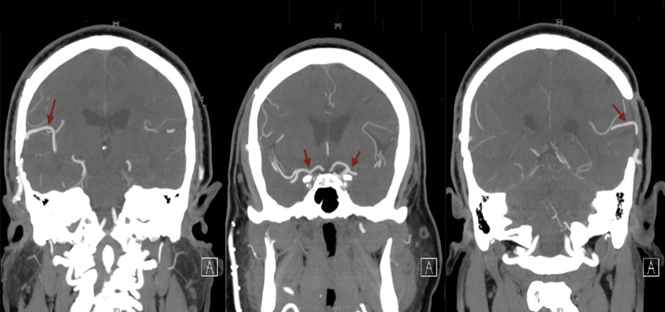
The patient described in this case underwent a successful left-sided superficial temporal artery-middle cerebral (STA-MCA) bypass in 2006. He was started on aspirin 81 mg daily. One year post-operatively (in 2007), DCA demonstrated a patent bypass and retrograde filling of the left MCA and ACA (Figure 2), and he had no ischemic symptoms that localized to that hemisphere.
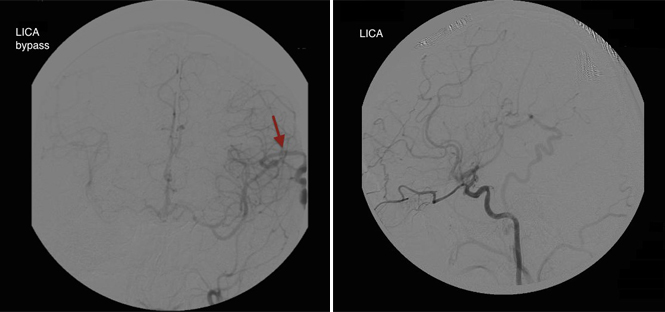
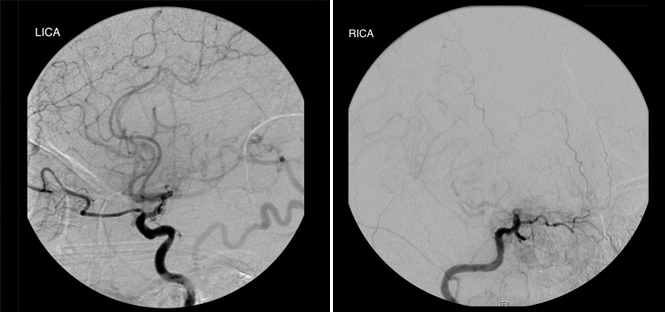
Three years post-operatively (in 2009), the patient began experiencing intermittent left hand and foot numbness concerning for transient ischemic attacks affecting the right hemisphere. DCA demonstrated adequate filling of the left ICA but worsened stenosis of the right supraclinoid ICA. He underwent a successful right-sided STA-MCA bypass and the aspirin was continued. CT angiography in 2010 demonstrated patent bilateral bypasses and retrograde filling of bilateral ACA’s and MCA’s (Figure 3). He continues to do well and is currently asymptomatic with no ischemic symptoms or neurological deficits (Figure 4).
ECIC bypass video from AANS Neurosurgeon on Vimeo.
References
1. Kim JS. Moyamoya disease; Epidemiology, clinical features, and diagnosis. Journal of Stroke. 2016;18(1):2-11.
[aans_authors]
THE EXPERTS WEIGH IN
Michael Schneck, md, Maywood, ill.
 MMD is a cerebrovascular disease characterized by bilateral proximal intracranial stenosis progressing to occlusion of the large intracranial arterial basal segments at the circle of Willis leading to development of arterial collateralization via small subcortical branches. MMD is associated with a higher incidence in East Asian children, with a possible familial occurrence suggesting a genetic component to the disease. This ‘basal occlusive disease’ (another term for MMD) can occur, however, in both children and adults worldwide and may be associated with other conditions, developing as a secondary response in the context of large vessel arteriopathy due to various causes.
MMD is a cerebrovascular disease characterized by bilateral proximal intracranial stenosis progressing to occlusion of the large intracranial arterial basal segments at the circle of Willis leading to development of arterial collateralization via small subcortical branches. MMD is associated with a higher incidence in East Asian children, with a possible familial occurrence suggesting a genetic component to the disease. This ‘basal occlusive disease’ (another term for MMD) can occur, however, in both children and adults worldwide and may be associated with other conditions, developing as a secondary response in the context of large vessel arteriopathy due to various causes.
MMD is typically reserved for pediatric patients with no other etiologic basis, and MMD is reserved for patients with the typical angiographic patterns in the context of other associated conditions. The most common associated conditions in children are neurofibromatosis type 1, cranial irradiation, Down syndrome and sickle cell disease. In adults, however, a wide variety of etiologic factors may contribute to acquired basal occlusive disease. Among the more common factors leading to acquired MMD in adults are accelerated atherosclerotic vascular disease, autoimmune vasculitidies, some hematologic disorders, post-infectious arteriopathy, post–subarachnoid or cranial trauma vasculopathy and sickle cell disease. There has even been a suggestion that oral contraceptives may increase the risk.
In children, ischemic cerebrovascular events, either transient ischemic attack or cerebral infarction, are predominate, whereas adults are more likely to present with either ischemic or hemorrhagic events. Patients of East Asian origin are at greater risk for hemorrhagic events as compared with patients in Western countries. Recurrent rates are variable with about half of patients having a recurrent stroke. Interestingly, ischemic events have been observed in children with crying, coughing, straining, fever, exercise or even hyperventilation. Whether similar stimuli that might affect cerebral blood flow or intracranial pressure are associated with an increased stroke risk in adults is unclear. Understanding these underlying risk profiles is important to vascular neurologists in determining the management of MMD patients.
The classical appearance of a puff of smoke reflecting compensatory development of small vessel collaterals is typically seen on conventional angiography, but the pattern of stenosis or occlusion of the intracranial carotid arteries, middle cerebral arteries or anterior cerebral arteries is well visualized on either MR or CT angiography. Generally, a MMD pattern is first identified by MRI in the context of a diagnostic evaluation for stroke or TIA, though sometimes it is found as part of an incidental finding (i.e. during evaluation of headache). That diagnostic MRI should include diffusion-weighted images to identify ischemia and gradient echo (T2*) or susceptibility-weighted (SWI) sequences for micro-hemorrhages. When structural imaging suggests an MMD pattern, cervicocerebral vessel imaging is mandatory. This does not mean that a conventional catheter angiogram is immediately required and an over-reliance on diagnostic catheter angiography is unnecessary. That is not to imply, however, that patients with an MMD pattern should not undergo a conventional angiogram. Rather, catheter angiography should be reserved for the purpose of surgical planning.
Prior to catheter angiography, to assess stroke risk and the potential response to a revascularization procedure, we routinely obtain an assessment of cerebral hemodynamic reserve. In the past, we have relied on either Xenon-CT or single photon emission computed tomography (SPECT) accompanied by an acetazolamide challenge to measure vasomotor reserve. While Xenon-CT is more quantitative than SPECT imaging, it is less readily available. Other modalities, such as oxygen-positron emission tomography to measure cerebral metabolic rates (CMRO2) might also be helpful as well but again, are not readily available. With the advent of new MR tissue perfusion techniques, such as hypercarbic blood oxygenation level dependent (BOLD) MRI sequences to measure cerebral vasoreactivity, hemodynamic measurements may become more readily available to assess tissue at risk and the response to revascularization. This would be preferable because an acetazolamide challenge to measure vasomotor reactivity theoretically can actually precipitate an ischemic event.
The acute management of an MMD patient with whom has sustained a stroke is largely supportive, involving control of intracranial pressure, seizure prevention and measures to maintain or improve cerebral blood flow. For ongoing care, however, referral to a center with a vascular neuroscience team, including both a vascular neurosurgeon and vascular neurologist, is essential. Low-dose aspirin therapy may be appropriate for primary and secondary stroke prevention in children as well as adults with cerebral ischemic events, as these patients are at high risk of subsequent ischemic events. Whether to treat asymptomatic adults with even low-dose aspirin, given the greater risk of hemorrhage in the adult population, is unknown.
All patients should have an extensive search for an underlying etiology and aggressive treatment of risk factors is indicated. Thus, for example, patients with atherosclerotic disease should also be started on statin therapy, and patients with sickle cell disease should probably undergo exchange transfusions. When patients with poor hemodynamic reserve, whether symptomatic or asymptomatic, are identified, early referral for either direct and/or indirect revascularization comes into play as untreated patients are at risk for recurrent ischemic and hemorrhagic stroke. The crucial consideration is that MMD patients should be managed as soon as the pathology is discovered at a center with a multidisciplinary team.
Michael J. Schneck, MD, is a professor of neurology and neurological surgery at Loyola University Medical Center/Stritch School of Medicine. He has a particular interest in vascular neurology and cerebral ischemia.
RESULTS FROM PREVIOUS GRAY MATTERS SURVEY
Topic: Treatment Options for Patient with MMD
[aans_poll id=”2679″ view=”results”]